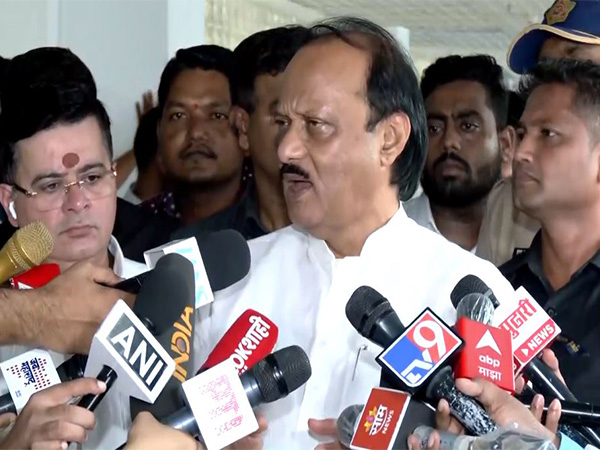Maharashtra govt writes to SII, Bharat Biotech about their demand of COVID-19 vaccines
Apr 28, 2021

Mumbai (Maharashtra) [India], April 28 : The Maharashtra government has written to COVID-19 vaccine manufacturers Serum Institute of India (SII) and Bharat Biotech about the state's vaccination requirements ahead of the next phase of the inoculation drive which starts on May 1.
While speaking about Maharashtra's vaccination drive for people between the ages of 18 to 45, state health minister Rajesh Tope on Tuesday said that about 12 crore vaccines would be required to fully vaccinate the adult population.
"We have over five crore people in the state who are 18 years of age and above. For the vaccination drive that will take place from May 1, we need about 12 crore vaccines. We have written to Serum Institute of India and Bharat Biotech about our demands for vaccines for people above 18," Tope said.
Meanwhile, Deputy Chief Minister Ajit Pawar has informed that Chief Minister Uddhav Thackeray will soon make a decision over whether the state government will conduct the administration of vaccines free of cost to all residents above the age of 18 years.
Maharashtra remains one of the worst-COVID-affected states in the country. As many as 66,358 new cases, 895 deaths and 67,752 recoveries were reported on Tuesday. The total case tally stands at 44,100,85.
The Centre on April 19 had announced a ''liberalised'' policy, making all above 18 years of age eligible to get vaccinated from May 1. It has also allowed state governments and private hospitals to purchase vaccines from manufacturers.
As per the central government, manufacturers of Covid-19 vaccines will be free to supply 50 per cent doses to state governments and in the open market, for which they will have to make an advance declaration of the price before May 1.
India had started the COVID-19 vaccination drive on January 16 with two vaccines -- Covishield (Oxford-AstraZeneca's vaccine manufactured by SII) and Covaxin (manufactured by Bharat Biotech Limited).
The second phase of the COVID-19 vaccination drive to inoculate people above 60 years and those over 45 with comorbidities against the coronavirus began on March 1.
The third phase began on April 1 for all above 45 years of age. In the next phase beginning May 1, all above the age of 18 would be eligible to receive the shot.